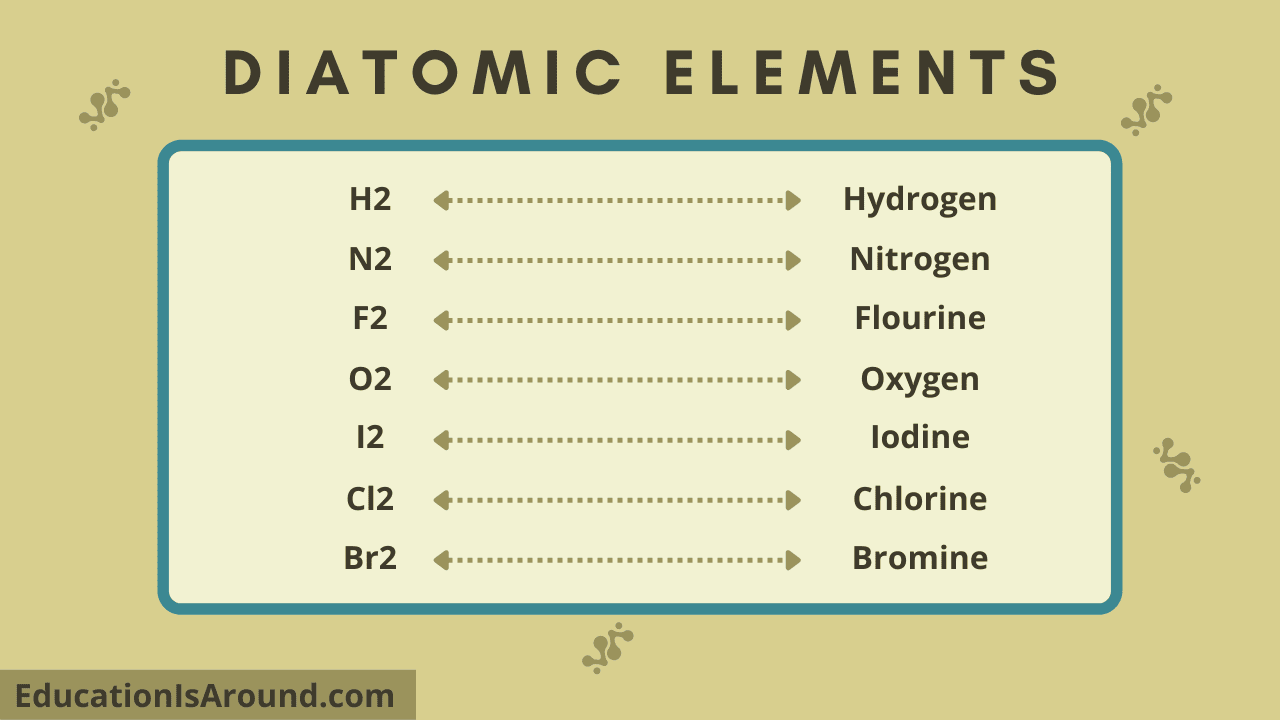
Does Boron Form A Diatomic Molecule . diatomic elements are pure elements that form molecules consisting of two atoms bonded together. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much.
from educationisaround.com
other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. diatomic elements are pure elements that form molecules consisting of two atoms bonded together.
Everything about Diatomic Elements Explained Education Is Around
Does Boron Form A Diatomic Molecule other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)).
From educationisaround.com
Everything about Diatomic Elements Explained Education Is Around Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. other. Does Boron Form A Diatomic Molecule.
From circuitvonalkau0.z19.web.core.windows.net
Lewis Electron Dot Diagram For Boron Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. Does Boron Form A Diatomic Molecule.
From www.vrogue.co
What Are The 7 Diatomic Elements Definition And List vrogue.co Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. Does Boron Form A Diatomic Molecule.
From www.mdpi.com
Molecules Free FullText Relative Stability of Boron Planar Does Boron Form A Diatomic Molecule other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where. Does Boron Form A Diatomic Molecule.
From www.vectorstock.com
Diagram representation of the element boron Vector Image Does Boron Form A Diatomic Molecule with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. other elements also exist naturally as diatomic molecules—a molecule with only two. Does Boron Form A Diatomic Molecule.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Does Boron Form A Diatomic Molecule Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. Does Boron Form A Diatomic Molecule.
From www.youtube.com
Atomic Structure (Bohr Model) for Boron (B) YouTube Does Boron Form A Diatomic Molecule other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is. Does Boron Form A Diatomic Molecule.
From borates.today
Alkaline Boron A Balancing Force In Nature Does Boron Form A Diatomic Molecule other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). diatomic elements are pure elements that form molecules consisting of two atoms bonded together. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. when two. Does Boron Form A Diatomic Molecule.
From www.shutterstock.com
Atomic Structure Lewis Dot Diagram Boron Stock Vector (Royalty Free Does Boron Form A Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). Valence bond theory. Does Boron Form A Diatomic Molecule.
From www.alamy.com
boron isotopes atomic structure backdrop physics theory illustration Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. Does Boron Form A Diatomic Molecule.
From circuitwiringhirsle77.z22.web.core.windows.net
Labelled Diagram Of Boron Atom Does Boron Form A Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. when two atomic orbitals combine to. Does Boron Form A Diatomic Molecule.
From www.chegg.com
Solved 7. The molecular orbital diagram for diatomic boron Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with. Does Boron Form A Diatomic Molecule.
From www.youtube.com
Diatomic Molecules and Covalent Bonding YouTube Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. Does Boron Form A Diatomic Molecule.
From material-properties.org
Boron Periodic Table and Atomic Properties Does Boron Form A Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with. Does Boron Form A Diatomic Molecule.
From www.dreamstime.com
Diatomic Molecules Elements Diagram Colors Stock Vector Illustration Does Boron Form A Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (table \(\pageindex{1}\)). Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with its high ionization energy, low. Does Boron Form A Diatomic Molecule.
From www.slideserve.com
PPT Chemistry 445. Lecture 4. Molecular Orbital Theory of diatomic Does Boron Form A Diatomic Molecule Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. Does Boron Form A Diatomic Molecule.
From medium.com
What is Boron? Periodic Table Elements Does Boron Form A Diatomic Molecule when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. with its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with. Does Boron Form A Diatomic Molecule.
From www.sciencephoto.com
Boron, atomic structure Stock Image C013/1499 Science Photo Library Does Boron Form A Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. when two atomic orbitals combine to form a pair of molecular orbitals, the bonding molecular orbital is stabilized about as much. Valence bond theory fails for a number of the second row diatomics, most famously for o 2, where it predicts a. with. Does Boron Form A Diatomic Molecule.